
|

Understanding through Discussion |
|
|
Register | Sign In |
|
QuickSearch
| EvC Forum active members: 64 (9164 total) |
|
| |
| ChatGPT | |
| Total: 916,839 Year: 4,096/9,624 Month: 967/974 Week: 294/286 Day: 15/40 Hour: 0/1 |
| Thread ▼ Details |
|
Member (Idle past 5060 days) Posts: 3428 From: Ithaca,NY, USA Joined: |
|
Thread Info
|
|
|
| Author | Topic: GP Gladyshev's paper (s)or mine? | |||||||||||||||||||||||
|
Brad McFall Member (Idle past 5060 days) Posts: 3428 From: Ithaca,NY, USA Joined: |
Gladyshev is correcting me-please wait--
from Moscow- From : ’ ‘?Sent : Monday, May 3, 2004 8:54 AM To : "Brad McFall" Subject : Re: Re:your papers and book on Thermodynamics and Evolution | | | Inbox Dear Brad, I have a look to "GP Gladyshev's :" site. Thank you ever so much! However, I would like to say that there is one mistake. In the second paragraph of your post (1451, 04-21-2004 10:49 AM) there is asentence: "The possible disagreement might (I dont know for sure) be traced to this quote". quote: e" Returning to the case of living creatures it may first be remarked that the making of an accurate entropy balance on an organism together with its environment:". It is your quote or Linschitz's quote (?)! This is not my quote! However, A LOT (?) (berberry, post: 309) decided that this is my quote. Hewrites: "That said, I fully concur with Gladyshev when he says: They then wish to go beyond (a),:.. ". I did not think so. I am sure the organisms do not avoid the second law! Asyou know, my statement is: Life in the Universe originated and is evolving in accordance with thegeneral laws of nature, specifically, the law of temporal hierarchies and the second law of thermodynamics. http://www.endeav.org/evolut/age/evol.htm I always say about the classical second law of thermodynamics (R. Clausius,J.W. Gibbs) only! As you know, there are many mistakes, which are connected with the interpretation of this law by L.Boltzmann, I.Prigogine and so on! I am not related to creationists! Thank you, Sincerely, Georgi PS. There is the mistake in my latter (04. 20. 2004) I sent you. It must behttp://www.endeav.org/evolut instead of http://www.endeav.org/evolute .
|
|||||||||||||||||||||||
|
berberry Inactive Member |
Brad,
Your note from Gladyshev refers to me and says, in response to my possible misinterpretation of Gladyshev's quote:
I did not think so. I am sure the organisms do not avoid the second law!
I agree, although I have no more reason to agree than my trust in science. I can't understand all of the concepts involved so I have to take it on trust. I extend this trust because I know that serious scientists base their opinions and conclusions on what can be observed, not what was written down by someone thousands of years ago. Gladyshev's statement:
They may then wish to go beyond (a), (b) and (c) and to assert that organisms somehow avoid the Second Law, even after allowing for their 'openness'.
was interpreted in my mind as saying that some creationists feel that the only explanation for life can be a supernatural one; i.e. one that does not obey the second law. Am I wrong here? It's quite possible that I still don't fully grasp the second law "controversy". If Gladyshev is actually reading this, may I suggest that he register here and post directly? He seems to understand you better than I do; he might be of some help to those of us who have trouble following your posts. Speaking of those posts, I'm still working on your last one. It might take me a while, but I'm determined to understand it at least to some degree. I can see that my (and others') inability to easily follow you is frustrating to you but I'm doing my best. You are MUCH smarter than I am; you have a knowledge of these concepts that I can't even approach. Please be patient. EDIT: In re-reading your post immediately above, it appears that the quote I originally responded to, then responded to again in this post, was yours, not Gladyshev's. Is that true? [This message has been edited berberry, 05-03-2004]
|
|||||||||||||||||||||||
|
Brad McFall Member (Idle past 5060 days) Posts: 3428 From: Ithaca,NY, USA Joined: |
berberry,
The quote was from Denbigh at GP's site. and was a page I had also read a few years ago when I firs started to get interested in macrothermodynamics. I guess I must have mislead you into thinking that this was Georgi's as well. Well I am sorry. The marginal notes that Georgi included shows me how I, BSM, dont need to take Denbigh word for word except in so far as chemical equilibria and some of the classics"" I am not schooled in. I think that in the past I had tried to read this page in order to try to understand Gladyshev better but concluded that if a creationist was in filiation with tne 2nd law and Gladyshev's law then one could INTERPRET Denbigh's use of the word "open" in ways that I now understand might be evidential for Wright's words such "parental longitude and latitude". At that time I had not been able to think of C/E issues back before 1900. I had used this quote in another place which signaled to me that it wasnt Georgi's but that was BEFORE I had recieved the papers from this outstanding scientist and I was still thinking that perhaps someone was playing a prank on me. I will do some work to make my latest long post more readable but I wanted the to keep up with internet time so I put it out as it was. I am not really any smarter. I have just thought about these things for more years perhaps. I think you read it correctly I had responded to Georgi in part with:>I did not think so. I am sure the organisms do not avoid the second law! As >you know, my statement is: I know that you do not think that. My "issue" is about what is 'open' and what closed. I am very excited to try to apply macrothermo to hox evo-devo evidence acquired since the 70s but the c-e context IS a little broader and LESS scientific"". I would have rather graduated from Cornell by trying to apply your equilibria to Wright's but this creationism thing seems to have gotten the better of the profs here. I know the difference very well because I "saw" it in my family with my gradfather and grandmother as non-believers and my mother and father as believers. This "split" continued somewhat down the generations to my 'level' with my brother in France believing least and my sister most. My brother Greg doesnt give YEC any credit and I only do because the creationists here in US were the only people who would talk to me about evolution once I was rejected at Cornell. Unfortunately after I contacted John Grehan (from New Zeland now in NY) he closed off e-mails perhaps because I HAD talked with the creationists. If every one recognized your "mechanism" then there would not be an issue but this would mean that people like Will Provine would be teaching the wrong dynamics(I am begining to think he gave Fisher too much credit but I have not verified this). I have no idea why he wanted to talk about the Jehova's Witness in a class titled "Evolution and Ethics" for I had thought that my family situation was somewhat exceptional and not the norm. >Life in the Universe originated and is evolving in accordance with the >general laws of nature, specifically, the law of temporal hierarchies and >the second law of thermodynamics. http://www.endeav.org/evolut/age/evol.htm > [This message has been edited Brad McFall, 05-03-2004]
|
|||||||||||||||||||||||
|
berberry Inactive Member |
In that very last paragraph, Brad, I think you have illuminated quite a bit that was dark to me. I'm still digesting it and I may have more to say and/or ask about it later. Got to get to work now.
|
|||||||||||||||||||||||
|
Brad McFall Member (Idle past 5060 days) Posts: 3428 From: Ithaca,NY, USA Joined: |
This is just to let you know that there was more than mere attribution issues going on...but it was more about info acquistion than any general entropy consequent
see David Z Albert TIME AND CHANCE Harvard 2000 Chapter5"The thing now is (obviously) to look for a catch. And the one thing that jumps right out at you in that connection is this issue of SENSITITIVTY, this fact that there can be no such thing as a Maxwellian demon that reliably END UP in ANY PARTICULAR MACROCONDITION...And insofar as I am aware, nothing whatsoever is currently known..."&"And there's something worth pausing over and taking note of here (by the way) in connection with the relationship between these PSEUDO-Maxwellian demons and bona fide Max...And as a matter of fact (if you stop and think about it) the considerations of Chapter 5 entail that their is nothing whatesoever in the classical equations of motion which stands in the way of there being (even) super-Maxwellian demons, which violate the second law on BOTH above conceptions of what the law is trying to say." You/one really need to read the full quotes or the whole book, just an FYI anyway [This message has been edited Brad McFall, 05-04-2004]
|
|||||||||||||||||||||||
|
berberry Inactive Member |
Brad,
I gather Will Provine, a professor under whom you studied, was responsible for your rejection from Cornell. When did that happen? Who is Fisher? My family is similar to yours in terms of religious faith. My mother is an avowed atheist, my father an Episcopalian (though not really a creationist as we know them here at evc). You've hinted that some creationists you know object to your reading the works of evolution scientists. How do you react to them when they raise their objections? I'll probably have more questions for you later, if you don't mind. If any of these questions are too personal for you then please ignore them. I'm only trying to get to know you a little better.
|
|||||||||||||||||||||||
|
berberry Inactive Member |
Brad McFall writes:
quote: What a clever metaphor! You should use the device more often, this one really helped my understanding.
quote: I'm almost scared to ask this, but since I don't really understand phenomenology I feel that I must. Please try to keep it simple: how is phenomenological thermodynamics distinguished from classic thermodynamics? I know that Gladyshev spoke (in the paper you posted) of a departure from classic thermodynamics which some scientists took during the 70s, but he seems to assume one already understands what the departure was. Was it a departure from classic TD to phenomenological TD? Also, does phonomenological TD have anything to do with creationism?
quote: That's because we want to know what your understanding is. As I said earlier, I realize this is frustrating for you, but some of us really do try to understand you. For my part, I wouldn't bother with all these questions if I didn't want to understand.
|
|||||||||||||||||||||||
|
Brad McFall Member (Idle past 5060 days) Posts: 3428 From: Ithaca,NY, USA Joined: |
I'll answer your other questions here later. Henry M. Morris simply said that whatever computer simulations or mathematical manipulations that evos could/can gain say would apply to creationism as well. I had suggested to him that creationists attempt to "predict" gaps not knowing that creatinists had already encounted evolutionists supprisingly claiming the that they are no longer looking for continuity but discontiniuty. This is all part of the the move away from equilibrium thinking that Gladyshev's priciple suffers by law as well I would guess. The logic of baraminology is distinctly more discreet than anything I have ever encountered or thought up in evolutionary theory.
RA Fisher is one of the 3 so-named "founders" of mathematical population genetics. Yes, Will Provine was ostensibly my "mentor" but though officially such in the College Scholar Program ( a special undergradute program at CU where one designs ones own education) he failed to take charge and out of time contraints I did but could not change the administrations view in the fall of '87 to reconsider it all once I waited to the last LEGAL day to petition for changes hoping that the profs could get back to the paper I wrote that they SIGNED ON to for me to DO. They never did so I tried to abort. The adminstrations did not like it that THEY had set such a date so far near the end of Semester but that was not my doing. I wrote the proposal and got 4 profs to sign it, including Will but they refused to honor (en mass) what they signed. When I challenged this a decade later I was escorted off campus by an undercover COP!!!!!!(I merely wanted to know into how many offices my files had spead!!). [This message has been edited Brad McFall, 05-04-2004]
|
|||||||||||||||||||||||
|
Brad McFall Member (Idle past 5060 days) Posts: 3428 From: Ithaca,NY, USA Joined: |
Your second question first,
I asked Jacques Derrida about science and Husserl and he said to talk to the scientists so I have never gone back to "transcentalism" (since around 1988) to address the issue of phenomenology on its own terms but in THE TRUTH IN PAINTING Derrida DID mention AMERICAN CREATIONISM. That would be a prerequiste before discussing any thermo in that content which IS suffiently structure in my view to contain any manipulation I have so far thought needed but nonexistant. That's my philosophical opnion but not a religious attitude. The answer to your first question is a little more difficult as you surmised. First, you can avoid the feeling that Gladyshev had it assumed the reader already understood the difference if you try to understand his position on Prigogine. In fact that is just a feeling from reading Russian in English. The "phenomenolgicality" is a consequence of realizing it necessary historically to seperate the kinetic"" approach of Prig from the tradition of thermo generally. If *heat* is explicitly worked into whatever system is open or closed as Prigogine suggested in some case then it might be possible to avoid that READING of Progine but if one inspects what Progine actually wrote FROM (the sources he cited) one finds that this is a stage BEYOND where Prig was at. I take it that GP has IN FACT gone beyond this. I had my own time trying to read Prig in the early 80s so I was already prepared to understand what GP was saying on that. As for a full histroy of the field of thermodynamics that is out of my knowledge base at the present time. I am aware of the difference between moderns like Wolfram and the classics like Clausius. I have been meaning to actually read Gibbs for some time now so for now I just take Georgi at his word that Gibbs has both a classical and phenomenological developement but I noted that perhaps Einstein might not have seen it this way. I dont know. In any case I have been interested in Gibbs because of his use of the word "perversion" so far and not as to the calculation of 'virials' that Einstein once mentioned perspicously but in passing. I hope this has not been too circuitous an answer. I have a few other things in mind but they will make the writing less clear than breakfast with collegues.
|
|||||||||||||||||||||||
|
Brad McFall Member (Idle past 5060 days) Posts: 3428 From: Ithaca,NY, USA Joined: |
This web site did not search and take your advice.
http://www.digisys.net/users/hoppnrmt/onlytheory.htm I am grateful that you interjected and provided an end that I might not have circuscripted otherwise in my first blush with Georgi's interest. I suspect this address more closely represents GP's view but since reading how Bertrand Russel TOOK the notion of an object IN CANTOR'S ADVANCE I can not be so certain that the Darwinization of Macrothermodynmics MUST contain any discontinuous systematic baramin no matter how poorly or inadequately defined and detailed to date. Bear linkages in this website do not conver the perhaps too many words that I have attempted in stead and It might in FACT BE that because with Hierarchical Thermodynamics THE GROUND that B.Russel DID NOT HAVE is a property now continuous in motion at least as to trends (grades/clades etc) that might first have been PERCEIVED by Agassiz. I dont know. I do think that Gould miswrote the relation of Paley and Agassiz however as to OGOD. I just dont thik that phenomenologically there need be ANY emergence here where the additivity of the classical approach egresses. I am not questioning everything on this site only the bottom
Wrong. Go back to the Evolution violates the 2nd of thermodynamics misconception and Classical Creationist Thermodynonsense.
where too much formatting seems to go to Prigogine. Materially Georgi solidified in my mind that there is TOO MUCH lip service to his work.
One of Wallace's own resources seems to find agreeable observations: Prigogine Center - "Although all real physical systems have a definite arrow of time, conventional quantum theory does not describe irreversible evolution in a fundamental fashion. Much of the on-going research at the Center is devoted to understanding how irreversible processes work, and if Dr. Prigogine's theory can furnish a mathematical description of these interesting processes.Mr. Wallace fails to show one paper, or shred of evidence where there is a universal dS < q/T caused by the evolutionary process. This violation is in his imagination unless the real physicists are doing their work all wrong. I chose the following from 500+ links found in a search of the word "evolution in The Institute of Physics : Entropic sampling and natural selection in biological evolutionDiffusion on a hypercubic lattice with pinning potential: exact results for the error-catastrophe problem in biological evolution Optimal self-organizatoin I was able to only find one link with creationism - Leslie J. Francis and John E. Greer, "Attitudes towards creationism and evolutionary theory: the debate among secondary pupils attending Catholic and Protestant schools in Northern Ireland Public Understanding of Science: Volume 8, Number 2, (93-103) There is also a workshop: Biological Evolution and Statistical Physics that will be held May 10-14, 2000 at the Max Plank Institute, where Mr. Wallace or his sources may want to present their compelling evidence. Also Check out these links: Track: Thermodynamics of Ontogenesis and Phylogenesis Differential equations of macrothermodynamics. The systems and the processes Hierarchical Thermodynamics
|
|||||||||||||||||||||||
|
Brad McFall Member (Idle past 5060 days) Posts: 3428 From: Ithaca,NY, USA Joined: |
In, "Thermodynamics of Biological Evolution and Aging" Georgi converged on the investigative requirement of the thermostat, in the same sense that Richard Lewontin asserts organisms "construct" their environment, namely in two paragraphs, "However, one must not forget that rational application of the methods of equilibrium thermodynamics in order to investigate the open systems under consideration needs certain limiting conditions to be fullfilled. One of the fundamental conditions is connected with the constancy (invariability) of the concentration of components of the solution or gas entering the column."
It definitely seems possible to me that Gould was mistaken how little Thompson's "direct imposition" was to figure in the hierarchicalization of the philosophy of biology. Gould made it quite clear he did not think what I am about to present could be recovered or discovered. He said,"...Cuvier acknowledged that science does not understand organic physics...and no one can imagine a mechanism for such globally coordinated alteration (Nor can one, even today, gainsay this excellent argument. If evolution were not mosaic, transmutation would be inconceivable, and would not occur precisely for the reasons stated by Cuvier." but I note the following two things of Agassiz on Cuvier:"I feel sure of success; the more so because Cuvier, who alone could do it (for the simple reason that every one else has till now neglected the fishes), is not engaged upon it."& and Humboldt to Coulon "I have strongly advised M. Agassiz not to accept the offers made to him at Paris since M. Cuvier's death, and his decision has anticipated my advice." Unfortunately Sedgwick was only able to think this through (Boole)sign wise. He wrote to Agassiz, "May God long preserve your life, which has been spent in promoting the great ends of truth and knowledge! Your great work on fossil fishes is now before me, and I posses the first number of your monograph upon the fishes of the Old Red Sandstone. I trust the new numbers will follow in rapid succession. I love now and then to find a resting-place; and your works always give me one. The opinions of Geoffroy St. Hillaire and his dark school seem to be gaining ground in England. I detest them, because I think them untrue. They shut out all argument from design and all notion of a Creative Providence, and in so doing they appear to me to deprive physiology of its life and strength, and language of its beauty and meaning. I am much offended in taste by the turgid mystical bombast of Geoffroy as I am disgusted by his cold and irrational materialism. When men of his school talk of the elective affinity of organic types, I hear jargon I cannot comphrehend, and I turn in disgust; and when they talk of spontaneous generation and transmutation of species, they seem to me to try nature by an hypothesis, and not to try their hypothesis by nature. Where are the facts on which to form an inductive truth? That's as far as Gould's imagination went. With phenomenological thermodyanmics we can think beyond these words. I contend that Agassiz's UNDERSTANDING of Old Red implies along with the more particular Cuvier determinant judgement , that the fossil layers less reflect hydrological sorting s as they do thermostat tolerance. This opens up a newly permitted chapter in the history of biology, for the modified thought of Aggasiz under invariance by way of Gladyshev's law, becomes surficially possible to ingrain adaptations not ONLY principally by natural selection, taxogenically but not necessarily cladistically. One must notice the limited extent of this evolution. It refers nomothetically only to the classifications that alingn vertebrates with the other (3)"types" not any new or old clade logic that also might have been anticipated panbiogeographically. I dont like writing outside my comfort zone but someone's got to do it. THE THERMOSTAT IS THE CONNOTATION OF THIS DENOTABLE MOSAIC!(so I, BSM, say today, in the biogeography of A's "prophetic types"). Darwin was mistaken only because he restricted his terminal classifications as dependent on articulations economically mirrorable but the 90s bubble burst into a new biological reality. This new segement of biotech continues to be marketed against the critcism any way. A responded to S with content Gould has already commented on, "I reproach myself for not acknowledging at once your most interesting letter of April 10th. But you will easily understand that in the midst of the rush of work consequent upon my preparation for a journey of several years' duration I have not noticed the flight of time since I received it, until to-day, when the sight of the date fills me with confusion. And yet, for years, I have not received a letter which has given me greater pleasure or moved me more deeply. I have felt in it and have received from it that vigor of conviction which gives to all you say or write a virile energy, captivating alike to the listener or the reader. Like you, I am pained by the progress of certain tendencies in the domain of the natrual sciences; it is not only the arid character of this philosophy of nature (and by this I mean, not natural philosophy, but "Natur-philosophie" of the Germans and French) which alarms me. My own personal interest is that I have been trying to figure out less how to build an organism from abiotic scratch than to extract energy from formal invariance. The reality of the thermostat enables one to focus particular physical bond schemes provided it is actually comprehended in the history of evolutionary thought. I have said here, I thought that Faraday and company demonstrated the first thermostat about the same time the dispute about evolution arose. Now I made a stronger claim that has historically been chalked up to accidental junk yard engineering. I didn't think so. If you don't think with me then you are thinking about metabolism and possibly false factually seperated replication and metabolism! If I am correct Freeman Dyson mde a major mistake. Organs but not organisms are bifurcated by thermostat levels of organization(still only quailitatively known 'parameterization'/boundary conditions) as to kinds. My first internet post (on Taxacom) is completely in line with Gladyshev's gas analogy but without the thermostat I had a more complicated construction based on virial properties. At first I did not realize this possibility taxogenically and simply thought the "solution" would remand ONLY Maxwell's electrotonics. This may not be so restricted. And for those still on the egg tooth here is some entertaining information that is psychologically connected across the means of communication of the same. "To this period belongs a curious dream mentioned by Agassiz in his work on the fossil fish. It is interesting both as a psychological fact and as showing how, sleeping and waking, his work ws ever present with him. He had been for two weeks striving to decipher the somewhat obscure impression of a fossil fish on the stone slab in which it was preserved. Weary and perplexed he put his work aside at last, and tried to dismiss it from his mind. Shortly after, he waked one night persuaded that while asleep he had seen his fish with all the missing features perfectly restored. But when he tried to hold and make fast the image, it escaped him. Nevertheless, he went early to the Jardin des Plantes, thinking that on looking anew at the impression he should see something which would put him on the track of his vision. In vain, - the blurred record was as blank as ever. The next night he saw the fish again, but with no more satisfactory result. When he awoke it disappeared from his memory as before. Hoping that the same experience might be repeated, on the third night he placed a pencil and paper besides his bed before going to sleep. Accordingly toward morning the fish reappeared in his dream, confusedly at first, but at last with such distinctness that he had no longer any doubt as to its zoological characters. This message has been edited by Brad McFall, 12-13-2004 03:43 PM This message has been edited by Brad McFall, 12-13-2004 04:03 PM
|
|||||||||||||||||||||||
|
Brad McFall Member (Idle past 5060 days) Posts: 3428 From: Ithaca,NY, USA Joined: |
I have more to say about the possible changes to Kantian Philosophy and the addition or not of the question of discontinuity systematics in the monoisolatable object on the better understanding of Chemistry that Georgi Gladyshev presents when quoting the classics as in this most recent communication from him below but I will let him speak more for himself. I will indicate the modifications if any in answer to Tony on the "quantum discretum"(sp?) of Kant to be edited into an already existing post.
Here is another "large quote" that perhaps indicated where we had some issues on the chemistry of life?
[Quote]
The Second Law of Thermodynamics and Evolutionof Living Systems Georgi P. GladyshevInternational Academy of Creative Endeavors, San Diego, USA Corresponding address: N. N. Semenov Institute of Chemical Physics, Russian Academy of Sciences, Kosygina 4, Moscow, 117977, Russia Abstract: The classical formulations of the second law of thermodynamics are presented. Some mistakes in the understanding the physical meaning of this general law of nature are noted. It is asserted that many misunderstandings of the second law of thermodynamics are related to terminological confusion and the underestimation of the theory developed by J.W. Gibbs and other founders of "true thermodynamics," which is impossible to disprove. To a certain approximation, R. Clausius and J.W. Gibbs' thermodynamics is applied to describing the evolution of living systems. This is possible due to the law of temporal hierarchies and the premise that the functions of state of living systems have real physical meaning at practically all hierarchical levels and at every moment of time. Making no pretensions to perfection, the author offers some advice to researchers dealing with thermodynamics. The author believes that, when considering thermodynamic problems, "ambiguous" terms and definitions should be clarified preliminarily in order to preclude possible misunderstandings. It is also advisable to refer to the classical works (including textbooks and encyclopedias) that the authors of publications have used. This will allow the correctness of the results reported to be estimated at least preliminarily. Keywords: Second law, thermodynamics, chemical thermodynamics, entropy, Gibbs function, Gibbs energy, full differential, law of temporal hierarchies, evolution, living systems, quasi-closed systems. AMS Mathematical Subject Classification: 74A15, 80-06, 80A50PACS: 05.70.-a, 64, 82.60.-s, 95.30.Tg " the true and only goal of science isto reveal unity rather than mechanisms" Henri Poincar Classics of science actually enunciated the second law of thermodynamics, one of general laws of nature, in the first half of the 19th century. Well-known formulations of this law are associated with the names of N.L.S. Carnot (1824), R. Clausius, (1850), and W. Thomson (Lord Kelvin) (1851). Although the formulations themselves are different, mainly because of the difference in phrasing, they may be considered equivalent. Many authors have attempted to change or improve the formulations as regards their physical meaning, yet none has succeeded. The meaning (essence) of these formulations has not been disproved to date [1—23]. However, new concepts have extended the possible applications of the second law of thermodynamics to different sciences, especially chemistry and, as it turned out later, biology. This became possible mainly due to J.W. Gibbs' works performed in 1873—1878. To a certain approximation, the methodology of Gibbs thermodynamics [2] has been extended to date to all hierarchies of natural systems, which are generally open ones [17]. The discovery of the law of temporal hierarchies, which may be considered a new general law of nature, has determined the extension of Gibbs's theory to living systems [17, 22—28]. This law [17] makes it possible to apply thermodynamics (more precisely, the hierarchic thermodynamics of quasi-closed systems), to all hierarchies of the real world, particularly, living objects and biological systems, to quite a good approximation. I believe that the discovery of this law confirms the universality of classical thermodynamic methods, and the name of Josiah Willard Gibbs even more vividly symbolizes the future of science that confirms the validity of general laws of nature as applied to the evolution of all material systems at all organizational levels of our world. Advances in classical thermodynamics (as well as approximate thermodynamics, i.e., the quasi-equilibrium thermodynamics of quasi-closed systems) are described in a number of textbooks and monographs. They are certainly numerous; I would especially recommend the works [1—17], which will be very useful for all beginner researchers.Clausius' formulation of the second law of thermodynamics, also known as the Clausius principle, states that a process that involves no changes except for the transfer of heat from a warmer body to a colder body is irreversible, i.e., heat cannot spontaneously pass from a colder body to a warmer one [7--10]. Clausius introduced the concept of entropy (S), a function of state of a system (a function that has a full differential). According to the Clausius inequality, dS δQ / T, (1) where the equality sign pertains to reversible processes and the inequality (greater-than) sign, to irreversible ones.Expression (1) is suitable for a simple isolated system, which can exchange neither substance nor energy with the environment and whose internal energy (U) and volume (V) are constant. In such systems only the work of expansion or no work at all is performed. In this case, the second law of thermodynamics may be written as dS 0 . Thus, the entropy of this system increases when irreversible processes occur, and it is maximum in the state of thermodynamic equilibrium.The second law of thermodynamics according to Thomson (Thomson's principle) states that the process during which work is transformed into heat without any other changes in the system's state is irreversible. This means that all heat withdrawn from a body cannot be entirely transformed into work unless the system is changed in other respects. This formulation is equivalent to the statement that the perpetuum mobile of the second kind is impossible [7-10]. Carnot's theorem is also equivalent to the impossibility of the perpetuum mobile of the second kind. According to this theorem, no heat engine can have a higher efficiency than that of the Carnot cycle, η = (T1 — T2)/T1, which is determined only by the temperatures of the heater and the cooler (T1 and T2, respectively). Carnot's theorem lays the basis for the absolute temperature scale. Sometimes, the second law of thermodynamics is formulated as the well-known C. Caratheodory's principle (1909). In the kinetic theory of gases, the second law of thermodynamics is substantiated by Boltzmann's H theorem. Here, H is the Boltzmann H function (to be precise, functional) determined from the mean logarithm of the particle distribution function. The Boltzmann H function is proportional to the entropy of the perfect gas. The physical meaning of entropy is revealed in statistical physics. Boltzmann demonstrated that the entropy is related to the logarithm of thermodynamic probability (W): S = k ln W, where k is the Boltzmann constant.Note that the Boltzmann's substantiating the statistical basis of the second law of thermodynamics, as well as the statistical substantiation of phenomenological thermodynamics suggested by Gibbs, involves ideal models, e.g., the perfect gas. In the case of more complex systems [3—4, 8], where pronounced (especially, strong) interactions between particles (molecules) are observed, it is difficult to perform the calculations. Therefore, it is obvious that these models are unlikely to be effective when studying most natural systems (e.g., biological), i.e., the systems that are far from corresponding to ideal or simple models. The thermodynamics of nonequilibrium processes deals with the rate of increase in or (which is the same) production of entropy. Therefore, it is sometimes asserted that nonequilibrium thermodynamics provides "the quantitative characteristic of the second law of thermodynamics" [7]. In the given case, however, this statement is reasonable only when applied to transformations in simple isolated systems where all processes are close to equilibrium. Only in a system that is close to equilibrium can the differential of this function of state of the system (entropy) be considered to be a full one, to an acceptable approximation. However, all the aforesaid is usually underestimated; therefore, many works on nonequilibrium thermodynamics, especially the thermodynamics of systems that are far from equilibrium, remain a faint "future hope." Some of these works, I daresay, are mere "mathematically trimmed" fantasies useless for real life [22—24]. Historically, the formulations of the second law of thermodynamics were closely associated with the study of heat engines. This approach has been developed by physicists, mainly thermal physicists, and heat engineers. Another trend in the use of the second law of thermodynamics is related to the attempts of some mathematicians and physicists constructing ideal and simple models to explain many natural phenomena in statistical terms. However, since all interactions in real systems are impossible to take into account, there is but little hope that calculations in the framework of these models will successfully solve the problem. Hence, only the phenomenological thermodynamics of systems close to equilibrium (equilibrium or quasi-equilibrium thermodynamics) is likely to ensure the insight into many natural phenomena and make reliable quantitative predictions. The above formulations of the second law of thermodynamics are, in a sense, somewhat outside the realm of the chemistry of molecular and supramolecular systems. These formulations may seem to be even farther from biology, sociology, and other sciences that are mainly based on chemistry (both molecular chemistry per se and the chemistry of supramolecular structures), which we perceive as "chemistry around us." Therefore, it is not unexpected that a purely physical (rather than physicochemical) approach to the origin of life, biological evolution, and aging of living organisms has lead to numerous misunderstandingsone might say, even to tragic errorsin life science. It should suffice to mention L. Boltzmann's, E. Schrdinger's, I. Progogine's [29—31], and other researchers' fallacies accounted for by neglecting (to some or another extent) Gibbs's works and underestimating the possibilities offered by thermodynamics. In some of my publications, I emphasized the substantial misunderstandings in this field [11, 20, 22, 23] that the founders of classical thermodynamics noted long ago [1, 2, 10—13]. To justify these statements, let me make a digression to cite the renowned scientists Boltzmann and Schrdinger [31] who asserted that living organisms struggle for negative entropy (also called negentropy). I will also cite some Prigogine's [29] quotations that appeared even on the cover of his books. The reader will find references to them in the Internet [31, 32]. I would like to note that the quotations presented below do not pertain to the second law of thermodynamics in its classical form [2, 9, 10]. Today, they may seem surprising, especially taking into account that all this was written several years after Gibbs published his works.For example, Boltzmann (1886) wrote, "The general struggle for existence of animate beings is therefore not a struggle for raw materials - these, for organisms, are air, water and soil, all abundantly availablenor for energy which exists in plenty in any body in the form of heat (albeit unfortunately not transformable), but a struggle for entropy, which becomes available through the transition of energy from the hot sun to the cold earth.". Then, in 1944, Schrdinger wrote that "the only way a living system stays alive, away from maximum entropy or death is to be continually drawing from its environment negative entropy. Thus the device by which an organism maintains itself stationary at a fairly high level of orderliness (= fairly low level of entropy) really consists in continually sucking orderliness from its environment. Plants of course have their most powerful supply in negative entropy in sunlight". Later, Prigogine also supposed (on the basis of previous notions of Boltzmann, Schrdinger, and their followers in life science) that the phenomenon of life is hardly consistent with the second law of thermodynamics. He noted, "During the last decades, an opinion has widely spread that there is the apparent contradiction between biological order and laws of physicsparticularly the second law of thermodynamics" (1980). Prigogine also emphasized that "this contradiction cannot be removed as long as one tries to understand living systems by the methods of equilibrium thermodynamics". It order to solve these "contradictions", Prigogine [29] developed the theory of dissipative structures, i.e., the structures that appearing in systems that are far from equilibrium. Later, it turned out that the theory did not allow overcoming the aforementioned "contradictions." In fact, it made the imbroglio even more intricate. It later became obvious that Prigogine's views do not agree with the second law of thermodynamics [20, 22, 24]. This is so in many respects. Suffice it to say that, in the general case, the Prigogine entropy (S' or S ) has no full differential. Therefore, his theory cannot be regarded as thermodynamic. This is a kinetic theory based on an "entropy" (Prigogine's entropy, S') which can be neither calculated nor measured. Prigogine considered Gibbs's work to be mainly theoretical and stated that Gibbs's method is inapplicable to studying physicochemical transformations, such as chemical reactions, because the values used in this method are the functions of state pertaining to the whole system or its individual component. Prigogine actively publicized his views via scientific literature and textbooks [30]. As a result, many researchers refer to Prigogine's formulation of the second law of thermodynamics. Actually, this formulation applies to a particular speculative model and may be accepted only on certain premises that cannot be proved. Unfortunately, this concept, which, in a sense, contradicts the principles of science itself [5, 6], was supported by many researchers. Owing to efficient publicity, these colleagues were convinced by hardly comprehensible (in physical terms) formulas and doubtful argumentation. In my opinion, the supporters of Prigogine's theory were, in a sense, deceived. Although Prigogine's theory proved an impasse, it still has its followers. Nevertheless, no numerical data obtained from either experiment or observation have confirmed the theory even at the qualitative level [20, 22]. Moreover, many physicochemical processes of the formation of spatially periodic structures (which Prigogine and his coauthors regarded as dissipative) were explained long ago in terms of the thermodynamic models of quasi-equilibrium systems (without involving the concept of dissipative structures). It is generally known that W.Ostwald (1897) used the notion of supersaturation to explain the existence of such systems in nature. The Second Law of Thermodynamics and Evolutionof Living Systems Georgi P. GladyshevInternational Academy of Creative Endeavors, San Diego, USA Corresponding address: N. N. Semenov Institute of Chemical Physics, Russian Academy of Sciences, Kosygina 4, Moscow, 117977, Russia Abstract: The classical formulations of the second law of thermodynamics are presented. Some mistakes in the understanding the physical meaning of this general law of nature are noted. It is asserted that many misunderstandings of the second law of thermodynamics are related to terminological confusion and the underestimation of the theory developed by J.W. Gibbs and other founders of "true thermodynamics," which is impossible to disprove. To a certain approximation, R. Clausius and J.W. Gibbs' thermodynamics is applied to describing the evolution of living systems. This is possible due to the law of temporal hierarchies and the premise that the functions of state of living systems have real physical meaning at practically all hierarchical levels and at every moment of time. Making no pretensions to perfection, the author offers some advice to researchers dealing with thermodynamics. The author believes that, when considering thermodynamic problems, "ambiguous" terms and definitions should be clarified preliminarily in order to preclude possible misunderstandings. It is also advisable to refer to the classical works (including textbooks and encyclopedias) that the authors of publications have used. This will allow the correctness of the results reported to be estimated at least preliminarily. Keywords: Second law, thermodynamics, chemical thermodynamics, entropy, Gibbs function, Gibbs energy, full differential, law of temporal hierarchies, evolution, living systems, quasi-closed systems. AMS Mathematical Subject Classification: 74A15, 80-06, 80A50PACS: 05.70.-a, 64, 82.60.-s, 95.30.Tg " the true and only goal of science isto reveal unity rather than mechanisms" Henri Poincar Classics of science actually enunciated the second law of thermodynamics, one of general laws of nature, in the first half of the 19th century. Well-known formulations of this law are associated with the names of N.L.S. Carnot (1824), R. Clausius, (1850), and W. Thomson (Lord Kelvin) (1851). Although the formulations themselves are different, mainly because of the difference in phrasing, they may be considered equivalent. Many authors have attempted to change or improve the formulations as regards their physical meaning, yet none has succeeded. The meaning (essence) of these formulations has not been disproved to date [1—23]. However, new concepts have extended the possible applications of the second law of thermodynamics to different sciences, especially chemistry and, as it turned out later, biology. This became possible mainly due to J.W. Gibbs' works performed in 1873—1878. To a certain approximation, the methodology of Gibbs thermodynamics [2] has been extended to date to all hierarchies of natural systems, which are generally open ones [17]. The discovery of the law of temporal hierarchies, which may be considered a new general law of nature, has determined the extension of Gibbs's theory to living systems [17, 22—28]. This law [17] makes it possible to apply thermodynamics (more precisely, the hierarchic thermodynamics of quasi-closed systems), to all hierarchies of the real world, particularly, living objects and biological systems, to quite a good approximation. I believe that the discovery of this law confirms the universality of classical thermodynamic methods, and the name of Josiah Willard Gibbs even more vividly symbolizes the future of science that confirms the validity of general laws of nature as applied to the evolution of all material systems at all organizational levels of our world. Advances in classical thermodynamics (as well as approximate thermodynamics, i.e., the quasi-equilibrium thermodynamics of quasi-closed systems) are described in a number of textbooks and monographs. They are certainly numerous; I would especially recommend the works [1—17], which will be very useful for all beginner researchers.Clausius' formulation of the second law of thermodynamics, also known as the Clausius principle, states that a process that involves no changes except for the transfer of heat from a warmer body to a colder body is irreversible, i.e., heat cannot spontaneously pass from a colder body to a warmer one [7--10]. Clausius introduced the concept of entropy (S), a function of state of a system (a function that has a full differential). According to the Clausius inequality, dS δQ / T, (1) where the equality sign pertains to reversible processes and the inequality (greater-than) sign, to irreversible ones.Expression (1) is suitable for a simple isolated system, which can exchange neither substance nor energy with the environment and whose internal energy (U) and volume (V) are constant. In such systems only the work of expansion or no work at all is performed. In this case, the second law of thermodynamics may be written as dS 0 . Thus, the entropy of this system increases when irreversible processes occur, and it is maximum in the state of thermodynamic equilibrium.The second law of thermodynamics according to Thomson (Thomson's principle) states that the process during which work is transformed into heat without any other changes in the system's state is irreversible. This means that all heat withdrawn from a body cannot be entirely transformed into work unless the system is changed in other respects. This formulation is equivalent to the statement that the perpetuum mobile of the second kind is impossible [7-10]. Carnot's theorem is also equivalent to the impossibility of the perpetuum mobile of the second kind. According to this theorem, no heat engine can have a higher efficiency than that of the Carnot cycle, η = (T1 — T2)/T1, which is determined only by the temperatures of the heater and the cooler (T1 and T2, respectively). Carnot's theorem lays the basis for the absolute temperature scale. Sometimes, the second law of thermodynamics is formulated as the well-known C. Caratheodory's principle (1909). In the kinetic theory of gases, the second law of thermodynamics is substantiated by Boltzmann's H theorem. Here, H is the Boltzmann H function (to be precise, functional) determined from the mean logarithm of the particle distribution function. The Boltzmann H function is proportional to the entropy of the perfect gas. The physical meaning of entropy is revealed in statistical physics. Boltzmann demonstrated that the entropy is related to the logarithm of thermodynamic probability (W): S = k ln W, where k is the Boltzmann constant.Note that the Boltzmann's substantiating the statistical basis of the second law of thermodynamics, as well as the statistical substantiation of phenomenological thermodynamics suggested by Gibbs, involves ideal models, e.g., the perfect gas. In the case of more complex systems [3—4, 8], where pronounced (especially, strong) interactions between particles (molecules) are observed, it is difficult to perform the calculations. Therefore, it is obvious that these models are unlikely to be effective when studying most natural systems (e.g., biological), i.e., the systems that are far from corresponding to ideal or simple models. The thermodynamics of nonequilibrium processes deals with the rate of increase in or (which is the same) production of entropy. Therefore, it is sometimes asserted that nonequilibrium thermodynamics provides "the quantitative characteristic of the second law of thermodynamics" [7]. In the given case, however, this statement is reasonable only when applied to transformations in simple isolated systems where all processes are close to equilibrium. Only in a system that is close to equilibrium can the differential of this function of state of the system (entropy) be considered to be a full one, to an acceptable approximation. However, all the aforesaid is usually underestimated; therefore, many works on nonequilibrium thermodynamics, especially the thermodynamics of systems that are far from equilibrium, remain a faint "future hope." Some of these works, I daresay, are mere "mathematically trimmed" fantasies useless for real life [22—24]. Historically, the formulations of the second law of thermodynamics were closely associated with the study of heat engines. This approach has been developed by physicists, mainly thermal physicists, and heat engineers. Another trend in the use of the second law of thermodynamics is related to the attempts of some mathematicians and physicists constructing ideal and simple models to explain many natural phenomena in statistical terms. However, since all interactions in real systems are impossible to take into account, there is but little hope that calculations in the framework of these models will successfully solve the problem. Hence, only the phenomenological thermodynamics of systems close to equilibrium (equilibrium or quasi-equilibrium thermodynamics) is likely to ensure the insight into many natural phenomena and make reliable quantitative predictions. The above formulations of the second law of thermodynamics are, in a sense, somewhat outside the realm of the chemistry of molecular and supramolecular systems. These formulations may seem to be even farther from biology, sociology, and other sciences that are mainly based on chemistry (both molecular chemistry per se and the chemistry of supramolecular structures), which we perceive as "chemistry around us." Therefore, it is not unexpected that a purely physical (rather than physicochemical) approach to the origin of life, biological evolution, and aging of living organisms has lead to numerous misunderstandingsone might say, even to tragic errorsin life science. It should suffice to mention L. Boltzmann's, E. Schrdinger's, I. Progogine's [29—31], and other researchers' fallacies accounted for by neglecting (to some or another extent) Gibbs's works and underestimating the possibilities offered by thermodynamics. In some of my publications, I emphasized the substantial misunderstandings in this field [11, 20, 22, 23] that the founders of classical thermodynamics noted long ago [1, 2, 10—13]. To justify these statements, let me make a digression to cite the renowned scientists Boltzmann and Schrdinger [31] who asserted that living organisms struggle for negative entropy (also called negentropy). I will also cite some Prigogine's [29] quotations that appeared even on the cover of his books. The reader will find references to them in the Internet [31, 32]. I would like to note that the quotations presented below do not pertain to the second law of thermodynamics in its classical form [2, 9, 10]. Today, they may seem surprising, especially taking into account that all this was written several years after Gibbs published his works.For example, Boltzmann (1886) wrote, "The general struggle for existence of animate beings is therefore not a struggle for raw materials - these, for organisms, are air, water and soil, all abundantly availablenor for energy which exists in plenty in any body in the form of heat (albeit unfortunately not transformable), but a struggle for entropy, which becomes available through the transition of energy from the hot sun to the cold earth.". Then, in 1944, Schrdinger wrote that "the only way a living system stays alive, away from maximum entropy or death is to be continually drawing from its environment negative entropy. Thus the device by which an organism maintains itself stationary at a fairly high level of orderliness (= fairly low level of entropy) really consists in continually sucking orderliness from its environment. Plants of course have their most powerful supply in negative entropy in sunlight". Later, Prigogine also supposed (on the basis of previous notions of Boltzmann, Schrdinger, and their followers in life science) that the phenomenon of life is hardly consistent with the second law of thermodynamics. He noted, "During the last decades, an opinion has widely spread that there is the apparent contradiction between biological order and laws of physicsparticularly the second law of thermodynamics" (1980). Prigogine also emphasized that "this contradiction cannot be removed as long as one tries to understand living systems by the methods of equilibrium thermodynamics". It order to solve these "contradictions", Prigogine [29] developed the theory of dissipative structures, i.e., the structures that appearing in systems that are far from equilibrium. Later, it turned out that the theory did not allow overcoming the aforementioned "contradictions." In fact, it made the imbroglio even more intricate. It later became obvious that Prigogine's views do not agree with the second law of thermodynamics [20, 22, 24]. This is so in many respects. Suffice it to say that, in the general case, the Prigogine entropy (S' or S ) has no full differential. Therefore, his theory cannot be regarded as thermodynamic. This is a kinetic theory based on an "entropy" (Prigogine's entropy, S') which can be neither calculated nor measured. Prigogine considered Gibbs's work to be mainly theoretical and stated that Gibbs's method is inapplicable to studying physicochemical transformations, such as chemical reactions, because the values used in this method are the functions of state pertaining to the whole system or its individual component. Prigogine actively publicized his views via scientific literature and textbooks [30]. As a result, many researchers refer to Prigogine's formulation of the second law of thermodynamics. Actually, this formulation applies to a particular speculative model and may be accepted only on certain premises that cannot be proved. Unfortunately, this concept, which, in a sense, contradicts the principles of science itself [5, 6], was supported by many researchers. Owing to efficient publicity, these colleagues were convinced by hardly comprehensible (in physical terms) formulas and doubtful argumentation. In my opinion, the supporters of Prigogine's theory were, in a sense, deceived. Although Prigogine's theory proved an impasse, it still has its followers. Nevertheless, no numerical data obtained from either experiment or observation have confirmed the theory even at the qualitative level [20, 22]. Moreover, many physicochemical processes of the formation of spatially periodic structures (which Prigogine and his coauthors regarded as dissipative) were explained long ago in terms of the thermodynamic models of quasi-equilibrium systems (without involving the concept of dissipative structures). It is generally known that W.Ostwald (1897) used the notion of supersaturation to explain the existence of such systems in nature. Thus, the aforementioned concepts by Boltzmann, Schrdinger, Prigogine, and their followers turned out to be at best tentative ones, or even a dead end. They hampered for many decades the search for the ways to explaining the evolution of living systems in physicochemical terms on the basis of the second law of thermodynamics. As noted above, only in recent decades were the principles of hierarchical thermodynamics (macrothermodynamics) formulated. I have managed to extend Gibbs's methodology so that it might be used for creating the physical (physicochemical) theories of the origin of life, biological evolution, and aging of living organisms [17, 22—28]. As noted above, the physical substantiation of the second law of thermodynamics deals with ideal processes and is based on the concept of statistical entropy. The nonequilibrium thermodynamics of systems that are far from equilibrium tries to study the changes in "kinetic entropy" (e.g., Prigogine's entropy S' or (S' or S ), which, as mentioned above, has no full differential (even an approximate one) and cannot be calculated in principle! In addition, the approaches used in the nonequilibrium thermodynamics of systems far from equilibrium create difficulties related, e.g., to the notions on the thermodynamics of processes and the thermodynamics of systems.One of the greatest merits of Gibbs and other renowned founders of classical thermodynamics is that they used the works by J.L. Lagrange, L. Euler, and other outstanding mathematicians (specifically, the variation principles developed by them) as a basis for the concepts on the functions of state of the system other than entropy (which, like entropy, have full differentials). The functions of state permit determining the directions of spontaneous processes and estimating the extent of their advancement in individual thermodynamic systems identified in the real world. In other words, the evolution of systems themselves can now be studied, to a certain approximation, if certain natural (independent) variables are constant. The Gibbs function G (the Gibbs free energy or, more briefly, the Gibbs energy) can be used for studying equilibrium (quasi-equilibrium) processes and closed systems (the quasi-closed systems in which quasi-equilibrium transformations occur) at constant temperature and pressure. The Helmholtz function F (A) is applicable to studying these processes and systems at constant temperature and volume. Certainly, this is only true on the assumption that the functions of state (of the systems studied) have actual physical sense at any moment of time. This is true for systems close to equilibrium but not for those far from equilibrium. I would like to emphasize once more that the law of temporal hierarchies gives grounds for the use of the functions of state when the direction and the extent of advancement of the evolutionary processes that occur in quasi-closed systems are estimated at different hierarchical levels of living matter [17]. For clarity, let us make a digression on the law of temporal hierarchies. The law of temporal hierarchies makes it possible to identify quasi-closed thermodynamic systems (subsystems) within open biological systems and to study the individual development (ontogenesis) and evolution (phylogenesis) of these subsystems via studying the changes in the specific (calculated per unit volume or mass) Gibbs function for the formation of a given higher monohierarchical structure out of lower monohierarchical structures. For example, it has been found that the specific Gibbs function for the formation of supramolecular structures of biological tissues ( ) tends towards its minimum in the course of ontogenesis (as well as phylogenesis and evolution as a whole):, (2) where V is the volume of the system, m is the mass of the microvolumes identified, x, y, and z are coordinates, the symbol - means that is a specific value (i.e., calculated per unit macrovolume), and the symbol ~ emphasizes that the system is heterogeneous. Note that expression (2) implies that the intermolecular (supramolecular) interactions in all hierarchical structures of biological tissues (both intracellular and intercellular interactions) are taken into account. This is justified because the structural hierarchy does not necessarily coincide with the temporal one. For example, cells of some types do not divide; like organs, they age simultaneously with the body as a whole. However, for each supramolecular hierarchy (j—1), there exists a higher hierarchy (j+х) such that << , where and are the mean lifetimes (or lifespan) of the elementary structures of the respective structural hierarchies in the living system; х = 0, 1, 2, etc.Note that the internal medium and many fragments of nondividing cells are nevertheless renewed due to metabolism. The use of expression (2) actually means that, in the given case, the law of temporal hierarchies assumes the following form: << << << << << . (3) Here, ( ) is the mean lifetime of molecules (chemical substance) involved in metabolism in the body, ( ) is the mean lifetime of all intermolecular (supramolecular) structures of tissues renewed during individual growth and development, is the mean lifetime of individual organisms in a population, and is the mean population lifetime. I have deliberately excluded the lifetimes of cells and some other complex supramolecular structures from the series of strong inequalities (3) for the reasons indicated above. However, this series certainly represents a general law of nature consistent with reality and reflecting the existence of temporal hierarchies in living systems.The law of temporal hierarchies is related to the presence of metabolism or other forms of substance transformation at all hierarchical levels. Note that metabolism is an essential characteristic of living organisms. This law (Gladyshev's law) allows strict demonstration of the possibility to identify (discern) quasi-open monohierarchical systems (subsystems) within open polyhierarchical biological systems. This statement entirely agrees with the experience accumulated in theoretical and experimental physics [3, 5]. I am convinced that this assertion cannot raise any objections. It is impossible in this short article to list all important conditions for the use of some or another function of state of the system. Moreover, I do not think I have noted all of the main "delicate" points that beginners should take into account. Besides, I refer to just a few publications, those that are most important for me. It should also be noted that my paper, as well as most publications on thermodynamics, may contain some inaccuracies of wording resulting from the ambiguity of translation. For example, most professional scientists know about inexcusable confusions with the terms isolated system and closed system (originally English). Both terms are sometimes translated into Russian as замкнутая система (literally, closed system). So the terms are often regarded as equivalent or identical. Other errors result from semantic coincidence of some terms. For example, the Gibbs (or Helmholtz) free energy is often confused with energy in the ordinary sense. This is why many researchers have attempted to replace this term with the term the Gibbs function [19]. Another example is the term complex system. Here, the word complex has a double meaning. In thermodynamics, a complex system (as opposed to a simple one) usually means a system in which (or on which) a work other than the work of expansion is done [15, 16]. Sometimes, however, the word complex is used to emphasize a structural or some other heterogeneity of the system itself or the diversity of its elements. This also applies to the term simple system, and so on. Certainly, these and other such confusions may lead to blunders that escape a nonprofessional's notice. These and other similar errors creep into some textbooks, reference books, and then into the Internet. I presume the possible inaccuracy of my English in this paper is insignificant, and I hope that the above remarks will warn beginners about the erroneous views that may exist in thermodynamics. I think that all physicists, chemists, biologists, and other specialists that deal with thermodynamics should study the Gibbs phenomenological thermodynamics first of all. As noted above, this authentic (in a certain sense, true) thermodynamics is based on the notion of full differentials. Note that this approach to understanding the world surrounding us is intrinsically irrefutable. We may only discuss the accuracy of the Gibbs thermodynamics as applied to, e.g., quasi-closed systems the processes in which are close to equilibrium. In accord with the very essence of full differential (its mathematical meaning), as well as the first law of thermodynamics, the change in the function of state of the system accompanying the transition from one equilibrium state to another is independent of the way or mechanisms of this transition. Probably, the lack of our knowledge on actual complex systems may be partly attributed to the changes in entropy during this transition, because the entropy cannot be measured directly. The changes in phenomenological entropy accompanying transformations in both simple and complex systems may be calculated only if one has studied the corresponding thermal processes. In statistical terms, the entropy is calculated only for ideal systems (or systems close to ideal). It is impossible to perform any precise calculations of this function of state for systems with strong interactions between particles (molecules and supramolecular structures) on a statistical basis. I would like to emphasize that this applies to complex thermodynamic systems, i.e., the systems in which strong interactions occur.Thermodynamics, owing to its impeccably reliable mathematical basis, may be regarded as a "machine" that always yields the right result if the premises are correct. Physical chemistry has repeatedly confirmed this [8—10, 14, 19]. Unfortunately, some physicists, biophysicists, biologists, and, especially, modern "philosophers" are still unaware of this experience of chemists and chemical technologists. I would like to repeat that the aforementioned ambiguities, which are mainly related to the underestimation of the correct use of many terms that are semantically similar but differ in physical meaning, result in confusion and misunderstandings. These misunderstandings discredit, at least in nonprofessionals' opinion, thermodynamics itself and science as a whole. Hence the numerous incorrect interpretations of the second law of thermodynamics, various dubious "views" on entropy [11, 13, 20, 22], and far-fetched "the functions of state of the system." Many authors, ignoring classical works in this field, apply different formulations of the second law of thermodynamics to systems where they are inherently inapplicable. Some of these authors suggest their own interpretations of this general law of nature. This debases science and education. Moreover, it can be said that several "second laws of thermodynamics" have appeared, none of which having anything to do with reality. A good example is the aforementioned Prigogine's [29] interpretation of the second law of thermodynamics. This interpretation "extends" the well-known incorrect and indemonstrable statement by the great Boltzmann [31], who underestimated the important concepts put forward by Clausius and Gibbs. The interpretation suggested by Prigogine has practically conquered the "scientific" world and still remains one of the trendiest interpretations of the second law of thermodynamics. I am well aware that it would be hopeless to argue with the visionaries that create or support these concepts: they have developed an excellent method for leading such debates. They unfailingly give lots of arguments, which are mostly quotations from published or oral statements made by other visionaries or by insufficiently informed scientists. It is often emphasized that those scientists are well known or even famous. However, the visionaries forget that scientists that are well known and famous in one field are not necessarily professionals in others. The only way to withstand this conjuncture is to refer the readers to classical works and serious textbooks written in a highly professional milieu of world-renowned scientific schools with centuries-long traditions. Thus, making no pretensions to perfection, I would like nevertheless to offer advice to researchers dealing with thermodynamics, as well as other branches of science, and the editors of scientific periodicals. My advice is the following. When discussing the problems of thermodynamics or using its mathematical tools for calculations, it is necessary to clarify "ambiguous" terms and definitions. It is also advisable to refer to the classical works (including textbooks, reference books, and encyclopedias) that the authors of publications used. In this case, the correctness of the results reported in the publications can be at least preliminarily estimated. I am grateful to Prof. M.M. Abdildin, Prof. G. Arrhenius, Prof. N.N. Bogolyubov, Jr., Prof. K.G. Denbigh, Prof. V.P. Kazakov, Prof. Yu.S. Lipatov, Prof. A.A. Logunov, Prof. V.V. Sychev, and Prof. V.M. Frolov for the support, systematic fruitful discussions, and valuable advice. Conclusion To a certain approximation, R. Clausius and J.W. Gibbs' thermodynamics is applied to describing the evolution of living systems. This is possible due to the law of temporal hierarchies and the premise that the functions of state of living systems have real physical meaning at practically all hierarchical levels and at every moment of time. The author believes that, when considering thermodynamic problems, "ambiguous" terms and definitions should be clarified preliminarily in order to preclude possible misunderstandings. It is also advisable to refer to the classical works (including textbooks and encyclopedias) that the authors of publications have used. References 1. A. Poincare’, On Science; Nauka: Moscow, 1983, 560 p.2. J.W. Gibbs, The Collected Works of J. Willard Gibbs. Thermodynamics; Longmans, Green and Co.: New York, 1928, V. 1, pp 55-349. 3. N.N. Bogolubov, Selected works. Part 1, Dynamical Theory; Gordon and Breach Science Publishers: New York, 1990. 4. N.N. Bogolubov and N.N. Bogolubov, Jr., An Introduction to Quantum Statistical mechanics; Gordon and Breach Science publishers: Library of Congress Catalog ISBN, 2-88124-879-9, QC 174.4.B6413 , 1992. 5. L.I. Sedov, The Thoughts on Science and on Scientists; Nauka, Russian Academy of Sciences, V.A. Steklov Mathematical Institute: Moscow, 1980, 440 p. 6. L.I. Sedov, Priroda - Russ., 1984, 11, 3. 7. D.N. Zubarev, The Carnot theorem, Physical Encyclopedia - Soviet Encyclopedia: Moscow, 1990; Vol. , pp. 242-243. 8. R.A Alberty, Physical Chemistry, 7th Ed.; Wiley: New York, Etc., 1987, 934 p. 9. R.J. Silbey, R.A Alberty, Physical Chemistry, 3rd Ed.; John Wiley & Sons: New York, 2001. 10. K.G. Denbigh, The Principle of Chemical Equilibrium, 3ed Ed.; Cambridge Univ. Press: Cambridge, 1971, 491 p. 11. K.G. Denbigh, Brit. J. Phyl. Sci. 1989, 40, 323. 12. K.G. Denbigh, Brit. J. Phil Sci. 1989, 40, 501. 13. K.G. Denbigh, J.S. Denbigh, Entropy in Relation to Incomplete Knowledge; Cambridge University Press: Cambridge, 1985. 14. Ch.R. Cantor, P.R. Schimmel, Biophysical Chemistry; Mir: Moscow, 1984-1985; Vol. 1-3. 15. V.V. Sychev, Thermodynamics of Complex Systems ; Energoatomizdat: Moscow, 1986, 208 p. 16. V.V. Sychev, Differential Equations of Thermodynamics ; Nauka: Moscow, 1981, 195 p. 17. G.P. Gladyshev, Supramolecular thermodynamics is a key to understanding phenomenon of life. What is Life from a Physical Chemist’s Viewpoint; Second Ed.; Regular and Chaotic Dynamics: Moscow — Izhevsk, 2003, 144 p. (In Russian). 18. Yu.S. Lipatov, Phase-Separated Interpenetrating Polymer Networks; USChTU (ISBN 966-8018-12) : Dnepropetrovsk, 2001, 326p. 19. M.N. Jones, Ed., Biochemical Thermodynamics; Elsevier Scientific Publishing Company: Amsterdam — Oxford — New York, 1979 (Russian translation. Mir: Moscow, 1982, 440 p.). 20. Shu-Kun Lin, Entropy, 1999, 1, 1 - http://www.mdpi.org/entropy 21. R.A. Alberty, Research Summary; Massachusetts Institute of Technology, Chemistry : Internet, 2004 MIT Department of Chemistry – Department of Chemistry at MIT 22. G.P. Gladyshev, International Journal of Modern Physics B, 2004, 18, 6, 801. 23 G.P. Gladyshev, Progress in Reaction Kinetics and Mechanism (An International Review Journal), 2003, 28, 157. 24. G.P. Gladyshev, Proceedings of International Higher Education Academy of Sciences, 2003, 4 (26), 19. 25. G.P. Gladyshev, Electron. J. Math. Phys. Sci., 2002, Sem. 2, 1 http://www.ejmaps.org 26. G.P. Gladyshev, SEED Journal (Canada), 2002, 3, 42, Error 404 | University of Toronto Libraries 27. G.P. Gladyshev, Entropy, 1999, 1, 2, 9 http://www.mdpi.org/entropy 28. G.P. Gladyshev, Entropy, 1999, 1, 4, 55 http://www.mdpi.org/entropy 29. I. Prigogine, From Being to Becoming: Time and Complexity and the Physical Sciences; W.H. Freeman & Co.: San Francisco, 1980. 30. I. Prigogine, R.Defey, Chemical Thermodynamics; Nauka: Novosibirsk, 1966, 509p. 31. References of works of L.Boltzmann, E.Schrdinger end I.Prigogine one can find in web-page http://www.redfish.com/...eiderKay1995_OrderFromDisorder.htm (E.D. Schneider, J.J. Kay, 1995, "Order from Disorder: The Thermodynamics of Complexity in Biology", in Michael P. Murphy, Luke A.J. O'Neill (ed), "What is Life: The Next Fifty Years. Reflections on the Future of Biology"; Cambridge University Press, pp. 161-172). 32. Information in Internet, The problems of Thermodynamics of Biological Evolution http://www.endeav.org/evolut As noted above, the physical substantiation of the second law of thermodynamics deals with ideal processes and is based on the concept of statistical entropy. The nonequilibrium thermodynamics of systems that are far from equilibrium tries to study the changes in "kinetic entropy" (e.g., Prigogine's entropy S' or (S' or S ), which, as mentioned above, has no full differential (even an approximate one) and cannot be calculated in principle! In addition, the approaches used in the nonequilibrium thermodynamics of systems far from equilibrium create difficulties related, e.g., to the notions on the thermodynamics of processes and the thermodynamics of systems.One of the greatest merits of Gibbs and other renowned founders of classical thermodynamics is that they used the works by J.L. Lagrange, L. Euler, and other outstanding mathematicians (specifically, the variation principles developed by them) as a basis for the concepts on the functions of state of the system other than entropy (which, like entropy, have full differentials). The functions of state permit determining the directions of spontaneous processes and estimating the extent of their advancement in individual thermodynamic systems identified in the real world. In other words, the evolution of systems themselves can now be studied, to a certain approximation, if certain natural (independent) variables are constant. The Gibbs function G (the Gibbs free energy or, more briefly, the Gibbs energy) can be used for studying equilibrium (quasi-equilibrium) processes and closed systems (the quasi-closed systems in which quasi-equilibrium transformations occur) at constant temperature and pressure. The Helmholtz function F (A) is applicable to studying these processes and systems at constant temperature and volume. Certainly, this is only true on the assumption that the functions of state (of the systems studied) have actual physical sense at any moment of time. This is true for systems close to equilibrium but not for those far from equilibrium. I would like to emphasize once more that the law of temporal hierarchies gives grounds for the use of the functions of state when the direction and the extent of advancement of the evolutionary processes that occur in quasi-closed systems are estimated at different hierarchical levels of living matter [17]. For clarity, let us make a digression on the law of temporal hierarchies. The law of temporal hierarchies makes it possible to identify quasi-closed thermodynamic systems (subsystems) within open biological systems and to study the individual development (ontogenesis) and evolution (phylogenesis) of these subsystems via studying the changes in the specific (calculated per unit volume or mass) Gibbs function for the formation of a given higher monohierarchical structure out of lower monohierarchical structures. For example, it has been found that the specific Gibbs function for the formation of supramolecular structures of biological tissues ( ) tends towards its minimum in the course of ontogenesis (as well as phylogenesis and evolution as a whole):, (2) where V is the volume of the system, m is the mass of the microvolumes identified, x, y, and z are coordinates, the symbol - means that is a specific value (i.e., calculated per unit macrovolume), and the symbol ~ emphasizes that the system is heterogeneous. Note that expression (2) implies that the intermolecular (supramolecular) interactions in all hierarchical structures of biological tissues (both intracellular and intercellular interactions) are taken into account. This is justified because the structural hierarchy does not necessarily coincide with the temporal one. For example, cells of some types do not divide; like organs, they age simultaneously with the body as a whole. However, for each supramolecular hierarchy (j—1), there exists a higher hierarchy (j+х) such that << , where and are the mean lifetimes (or lifespan) of the elementary structures of the respective structural hierarchies in the living system; х = 0, 1, 2, etc.Note that the internal medium and many fragments of nondividing cells are nevertheless renewed due to metabolism. The use of expression (2) actually means that, in the given case, the law of temporal hierarchies assumes the following form: << << << << << . (3) Here, ( ) is the mean lifetime of molecules (chemical substance) involved in metabolism in the body, ( ) is the mean lifetime of all intermolecular (supramolecular) structures of tissues renewed during individual growth and development, is the mean lifetime of individual organisms in a population, and is the mean population lifetime. I have deliberately excluded the lifetimes of cells and some other complex supramolecular structures from the series of strong inequalities (3) for the reasons indicated above. However, this series certainly represents a general law of nature consistent with reality and reflecting the existence of temporal hierarchies in living systems.The law of temporal hierarchies is related to the presence of metabolism or other forms of substance transformation at all hierarchical levels. Note that metabolism is an essential characteristic of living organisms. This law (Gladyshev's law) allows strict demonstration of the possibility to identify (discern) quasi-open monohierarchical systems (subsystems) within open polyhierarchical biological systems. This statement entirely agrees with the experience accumulated in theoretical and experimental physics [3, 5]. I am convinced that this assertion cannot raise any objections. It is impossible in this short article to list all important conditions for the use of some or another function of state of the system. Moreover, I do not think I have noted all of the main "delicate" points that beginners should take into account. Besides, I refer to just a few publications, those that are most important for me. It should also be noted that my paper, as well as most publications on thermodynamics, may contain some inaccuracies of wording resulting from the ambiguity of translation. For example, most professional scientists know about inexcusable confusions with the terms isolated system and closed system (originally English). Both terms are sometimes translated into Russian as замкнутая система (literally, closed system). So the terms are often regarded as equivalent or identical. Other errors result from semantic coincidence of some terms. For example, the Gibbs (or Helmholtz) free energy is often confused with energy in the ordinary sense. This is why many researchers have attempted to replace this term with the term the Gibbs function [19]. Another example is the term complex system. Here, the word complex has a double meaning. In thermodynamics, a complex system (as opposed to a simple one) usually means a system in which (or on which) a work other than the work of expansion is done [15, 16]. Sometimes, however, the word complex is used to emphasize a structural or some other heterogeneity of the system itself or the diversity of its elements. This also applies to the term simple system, and so on. Certainly, these and other such confusions may lead to blunders that escape a nonprofessional's notice. These and other similar errors creep into some textbooks, reference books, and then into the Internet. I presume the possible inaccuracy of my English in this paper is insignificant, and I hope that the above remarks will warn beginners about the erroneous views that may exist in thermodynamics. I think that all physicists, chemists, biologists, and other specialists that deal with thermodynamics should study the Gibbs phenomenological thermodynamics first of all. As noted above, this authentic (in a certain sense, true) thermodynamics is based on the notion of full differentials. Note that this approach to understanding the world surrounding us is intrinsically irrefutable. We may only discuss the accuracy of the Gibbs thermodynamics as applied to, e.g., quasi-closed systems the processes in which are close to equilibrium. In accord with the very essence of full differential (its mathematical meaning), as well as the first law of thermodynamics, the change in the function of state of the system accompanying the transition from one equilibrium state to another is independent of the way or mechanisms of this transition. Probably, the lack of our knowledge on actual complex systems may be partly attributed to the changes in entropy during this transition, because the entropy cannot be measured directly. The changes in phenomenological entropy accompanying transformations in both simple and complex systems may be calculated only if one has studied the corresponding thermal processes. In statistical terms, the entropy is calculated only for ideal systems (or systems close to ideal). It is impossible to perform any precise calculations of this function of state for systems with strong interactions between particles (molecules and supramolecular structures) on a statistical basis. I would like to emphasize that this applies to complex thermodynamic systems, i.e., the systems in which strong interactions occur.Thermodynamics, owing to its impeccably reliable mathematical basis, may be regarded as a "machine" that always yields the right result if the premises are correct. Physical chemistry has repeatedly confirmed this [8—10, 14, 19]. Unfortunately, some physicists, biophysicists, biologists, and, especially, modern "philosophers" are still unaware of this experience of chemists and chemical technologists. I would like to repeat that the aforementioned ambiguities, which are mainly related to the underestimation of the correct use of many terms that are semantically similar but differ in physical meaning, result in confusion and misunderstandings. These misunderstandings discredit, at least in nonprofessionals' opinion, thermodynamics itself and science as a whole. Hence the numerous incorrect interpretations of the second law of thermodynamics, various dubious "views" on entropy [11, 13, 20, 22], and far-fetched "the functions of state of the system." Many authors, ignoring classical works in this field, apply different formulations of the second law of thermodynamics to systems where they are inherently inapplicable. Some of these authors suggest their own interpretations of this general law of nature. This debases science and education. Moreover, it can be said that several "second laws of thermodynamics" have appeared, none of which having anything to do with reality. A good example is the aforementioned Prigogine's [29] interpretation of the second law of thermodynamics. This interpretation "extends" the well-known incorrect and indemonstrable statement by the great Boltzmann [31], who underestimated the important concepts put forward by Clausius and Gibbs. The interpretation suggested by Prigogine has practically conquered the "scientific" world and still remains one of the trendiest interpretations of the second law of thermodynamics. I am well aware that it would be hopeless to argue with the visionaries that create or support these concepts: they have developed an excellent method for leading such debates. They unfailingly give lots of arguments, which are mostly quotations from published or oral statements made by other visionaries or by insufficiently informed scientists. It is often emphasized that those scientists are well known or even famous. However, the visionaries forget that scientists that are well known and famous in one field are not necessarily professionals in others. The only way to withstand this conjuncture is to refer the readers to classical works and serious textbooks written in a highly professional milieu of world-renowned scientific schools with centuries-long traditions. Thus, making no pretensions to perfection, I would like nevertheless to offer advice to researchers dealing with thermodynamics, as well as other branches of science, and the editors of scientific periodicals. My advice is the following. When discussing the problems of thermodynamics or using its mathematical tools for calculations, it is necessary to clarify "ambiguous" terms and definitions. It is also advisable to refer to the classical works (including textbooks, reference books, and encyclopedias) that the authors of publications used. In this case, the correctness of the results reported in the publications can be at least preliminarily estimated. I am grateful to Prof. M.M. Abdildin, Prof. G. Arrhenius, Prof. N.N. Bogolyubov, Jr., Prof. K.G. Denbigh, Prof. V.P. Kazakov, Prof. Yu.S. Lipatov, Prof. A.A. Logunov, Prof. V.V. Sychev, and Prof. V.M. Frolov for the support, systematic fruitful discussions, and valuable advice. Conclusion To a certain approximation, R. Clausius and J.W. Gibbs' thermodynamics is applied to describing the evolution of living systems. This is possible due to the law of temporal hierarchies and the premise that the functions of state of living systems have real physical meaning at practically all hierarchical levels and at every moment of time. The author believes that, when considering thermodynamic problems, "ambiguous" terms and definitions should be clarified preliminarily in order to preclude possible misunderstandings. It is also advisable to refer to the classical works (including textbooks and encyclopedias) that the authors of publications have used. References 1. A. Poincare’, On Science; Nauka: Moscow, 1983, 560 p.2. J.W. Gibbs, The Collected Works of J. Willard Gibbs. Thermodynamics; Longmans, Green and Co.: New York, 1928, V. 1, pp 55-349. 3. N.N. Bogolubov, Selected works. Part 1, Dynamical Theory; Gordon and Breach Science Publishers: New York, 1990. 4. N.N. Bogolubov and N.N. Bogolubov, Jr., An Introduction to Quantum Statistical mechanics; Gordon and Breach Science publishers: Library of Congress Catalog ISBN, 2-88124-879-9, QC 174.4.B6413 , 1992. 5. L.I. Sedov, The Thoughts on Science and on Scientists; Nauka, Russian Academy of Sciences, V.A. Steklov Mathematical Institute: Moscow, 1980, 440 p. 6. L.I. Sedov, Priroda - Russ., 1984, 11, 3. 7. D.N. Zubarev, The Carnot theorem, Physical Encyclopedia - Soviet Encyclopedia: Moscow, 1990; Vol. , pp. 242-243. 8. R.A Alberty, Physical Chemistry, 7th Ed.; Wiley: New York, Etc., 1987, 934 p. 9. R.J. Silbey, R.A Alberty, Physical Chemistry, 3rd Ed.; John Wiley & Sons: New York, 2001. 10. K.G. Denbigh, The Principle of Chemical Equilibrium, 3ed Ed.; Cambridge Univ. Press: Cambridge, 1971, 491 p. 11. K.G. Denbigh, Brit. J. Phyl. Sci. 1989, 40, 323. 12. K.G. Denbigh, Brit. J. Phil Sci. 1989, 40, 501. 13. K.G. Denbigh, J.S. Denbigh, Entropy in Relation to Incomplete Knowledge; Cambridge University Press: Cambridge, 1985. 14. Ch.R. Cantor, P.R. Schimmel, Biophysical Chemistry; Mir: Moscow, 1984-1985; Vol. 1-3. 15. V.V. Sychev, Thermodynamics of Complex Systems ; Energoatomizdat: Moscow, 1986, 208 p. 16. V.V. Sychev, Differential Equations of Thermodynamics ; Nauka: Moscow, 1981, 195 p. 17. G.P. Gladyshev, Supramolecular thermodynamics is a key to understanding phenomenon of life. What is Life from a Physical Chemist’s Viewpoint; Second Ed.; Regular and Chaotic Dynamics: Moscow — Izhevsk, 2003, 144 p. (In Russian). 18. Yu.S. Lipatov, Phase-Separated Interpenetrating Polymer Networks; USChTU (ISBN 966-801
|
|||||||||||||||||||||||
|
Brad McFall Member (Idle past 5060 days) Posts: 3428 From: Ithaca,NY, USA Joined: |
It appeared to me, in light of Buzzsaw's conversation, that EVC has not been able to move continually forward becuase of lack of apprehension of Georgi's
quote:which in a prior time gave rise or had already arisen in my thought as below. 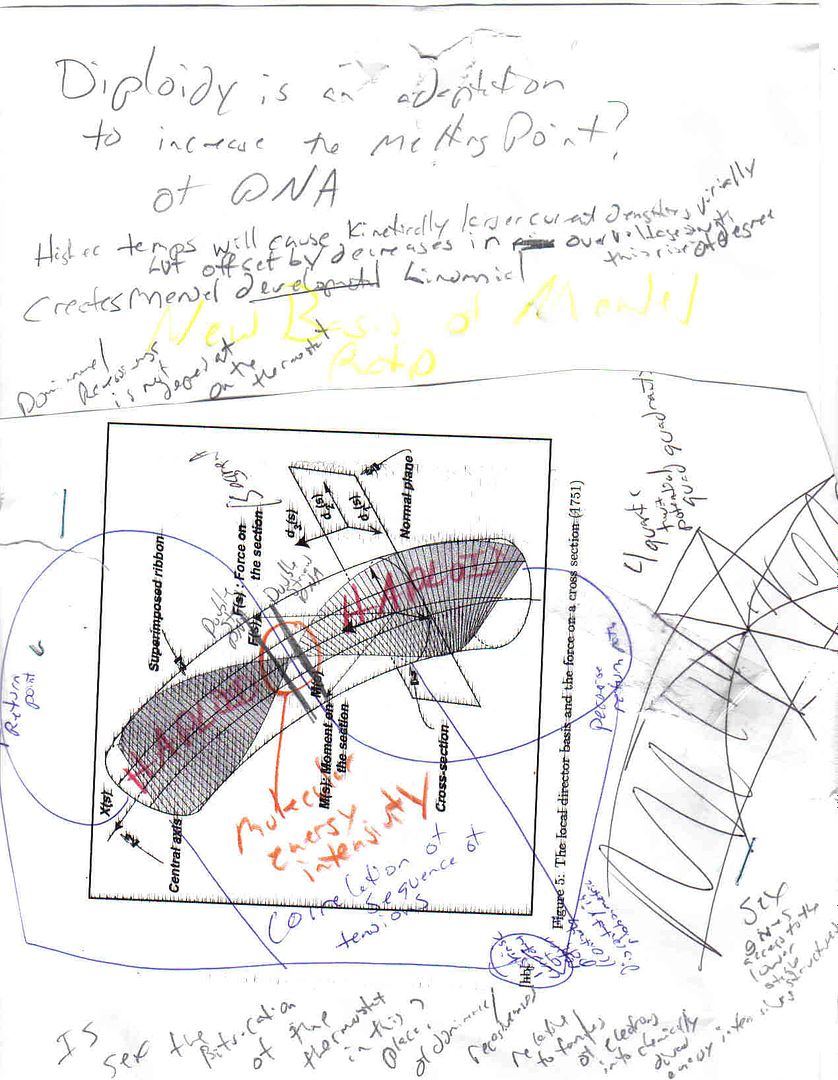 It should be possible for the reader to come to a different thought of the relation between these fundamental particles and a whole (word as expressed also before IN CLASS with Will Provine and ajudged to be part of a Freudian mental phenomenon, whether critically located correctly or not).
|
|||||||||||||||||||||||
Syamsu  Suspended Member (Idle past 5617 days) Posts: 1914 From: amsterdam Joined: |
I don't understand all of this. I thought Gladyshev's basic idea was that systems wear down, and that they constantly have to introduce something new, in order not to break down. This leaves the stronger structures more likely to be left over, but the point seems to be the newness that prevents collapse. Newness does not point to any law, obviously, so Gladyshev's affirmation of the universe behaving according to laws makes no sense.
regards,Mohammad Nor Syamsu
|
|||||||||||||||||||||||
|
Brad McFall Member (Idle past 5060 days) Posts: 3428 From: Ithaca,NY, USA Joined: |
I will have to describe more fully the differences and defintions of the following:
Phenomenological thermodynamicsMacrothermodynamics Hierarchical Thermodyanmics Macrokinetics http://mendcomm.org/index/institutes-matsci.html The Institute for Structural Macrokinetics, Russian Academy of Sciences (Russian acronym is ISMAN) is a young and developing academic institution in the field of macroscopic kinetics of chemical reactions. The researchers working at the Institute are interested in the processes of any chemical nature in which an important part is played by physical factors, e.g. processes of heat and mass transfer, phase and structural transformations. Theoretical and experimental studies of the mutual effect of chemical and physical processes, the revealing of direct and inverse relations between them and the description of phenomena, modes and effects due to these
I have suggested even MORE,That the unification of forces relied (past tense) on continuous HOMOLOGY between problems in attractions and in heat. This depends on the code lingo somewhat so what I have added might not exist while GG's classicaly does and did. The pictue I provide above indicates my own take on what BIOLOGICALLY the component Gibbs segement would be. This need not come out the same as Georgi's less qualitative presentation but the whole MUST and IS the same. That whole is what I learned by rereading in terms of his latest "mono" hierarchy. This result of his appears to have been determined by contrasted a poly volcal English USE of "isolated" and "closed" which are often univocal in Russian. I had been denoting things differently with these words but I take no credit for any of Dr. Gladyshev's insights. If I had to guess you might try focusing on his comments about transfers from higher levels and interference with the genetic transmission. I will try to find a few of these points for you. http://www.endeav.org/evolut/text/canada2001/index.htm
quote: I don't understand all of this. I thought Gladyshev's basic idea was that systems wear down, and that they constantly have to introduce something new, in order not to break down. This leaves the stronger structures more likely to be left over, but the point seems to be the newness that prevents collapse. Newness does not point to any law, obviously, so Gladyshev's affirmation of the universe behaving according to laws makes no sense. The thing to grasp is that the higher levels USE the less stable parts of the lower levels, given the discontinuity, through this 2nd law.
quote:The implication WAS that one could isolate in temporal biological cycles a subset of the genetic continuum. The qualitative presentation didnt say exactly what this was but there are indications relative to the upper thermal limit of proteins without melting etc. At firt I was questioning this conclusion but since find that there is not errant biologically not matter what the maths spell. I have visualized this process WITHIN topobiology and so I was able to think it WITHOUT Lewontin's notion of Scottish Economics which he relied on relative to any topology in the Triple Helix. The picture above is what I have variously written as a PERVERSION but I have located DNA not proteins ( the scribbled figure on the right was first thought up in terms of proteins before getting the Gladyshev correspondence). I will upload some more later but this will have to be for now.  on review of Gould's complaint about Dawkins' change from 76' to 82' I think your problem comes from the concept of PLUrIFACTION. I hope this helps. The necker cube can not be here. This message has been edited by Brad McFall, 01-24-2005 20:13 AM
|
|||||||||||||||||||||||
|
|
Do Nothing Button
Copyright 2001-2023 by EvC Forum, All Rights Reserved
 ™ Version 4.2
™ Version 4.2
Innovative software from Qwixotic © 2024