[QUOTE]Originally posted by TrueCreation:
[b]--Nice link, however do you have the variables handy which were used to calculate this? ie, do you have the data?[/QUOTE]
[/b]
Anbar, A. D., G. J. Wasserburg, D. A. Papanastassiou, and P. S. Andersson, Iridium in natural waters, Science , 273, 1524-1528, 1996
http://www.sciencemag.org/cgi/content/abstract/273/5281/1524
[QUOTE]--This is another nice link and a graph of the concentration of iridium, the format is interesting by which it decreases in quantity.
http://rainbow.ldeo.columbia.edu/courses/v1001/impact23.html
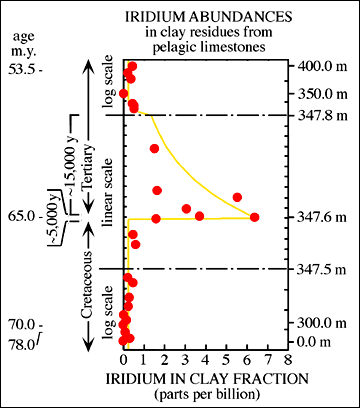
[/B][/QUOTE]
It seems to decay exponentially over tens of thousands of years, corraborating Anbar's numbers very nicely.


 ™ Version 4.2
™ Version 4.2